Do your CRF design and see exactly how it looks in ryze before building your EDC.
- Book a demo
- Free trial
- Solutions
ryze
ryze FEATURES
Formedix CORE
- Services
- About
- Resources
- Blog
- Contact
- Book a demo
- Free trial
Do your CRF design and see exactly how it looks in ryze before building your EDC.
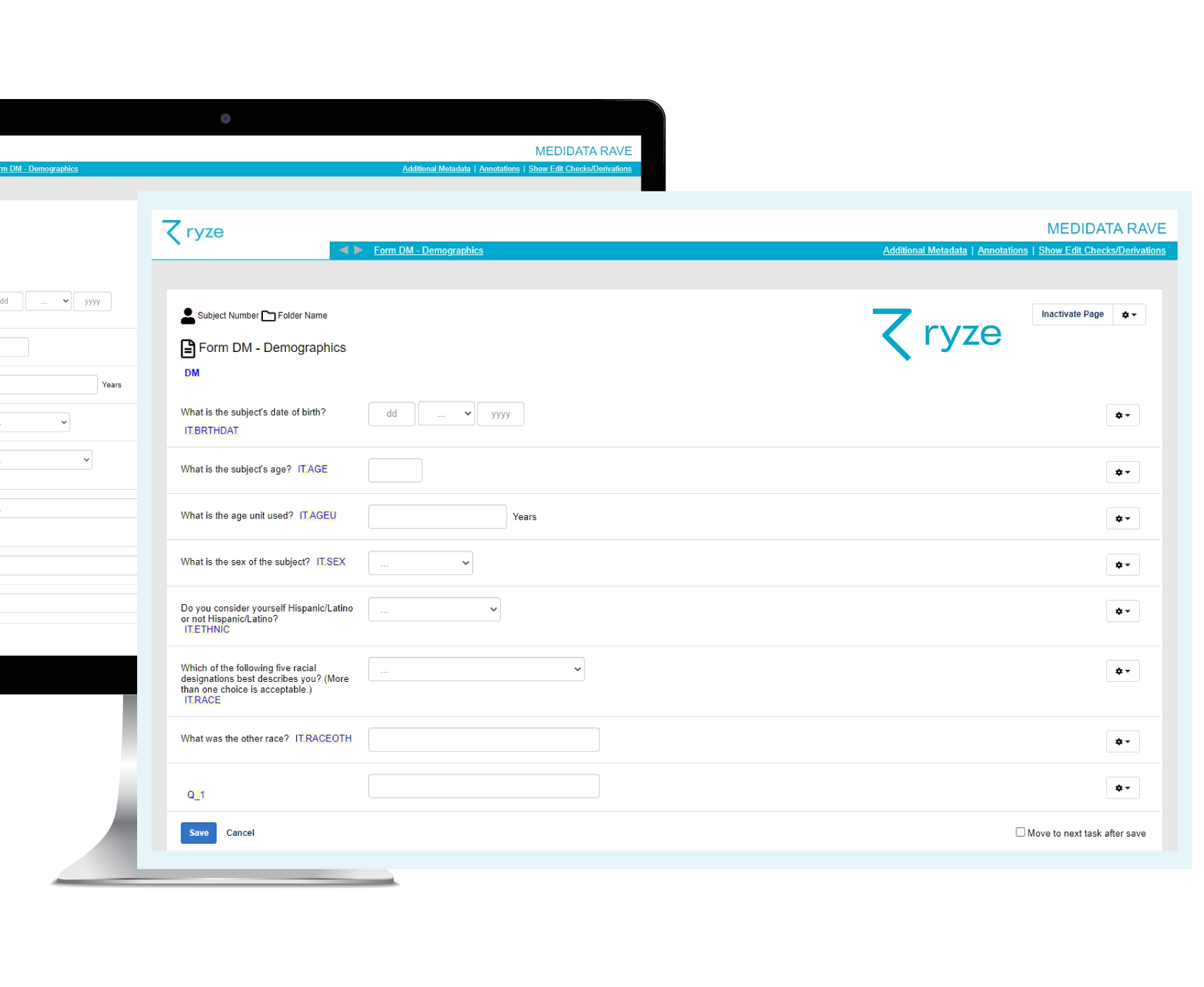
Do CRF designs in ryze and see exactly how forms look and work for leading EDCs – before building your study! That way you can gain feedback from stakeholders, make changes and preview designs in ryze until they’re approved before EDC build happens.
Once study CRFs are approved, it’s just one click to build your full EDC study from ryze. This includes all your EDC system specific functionality, such as edit checks and visit structures.
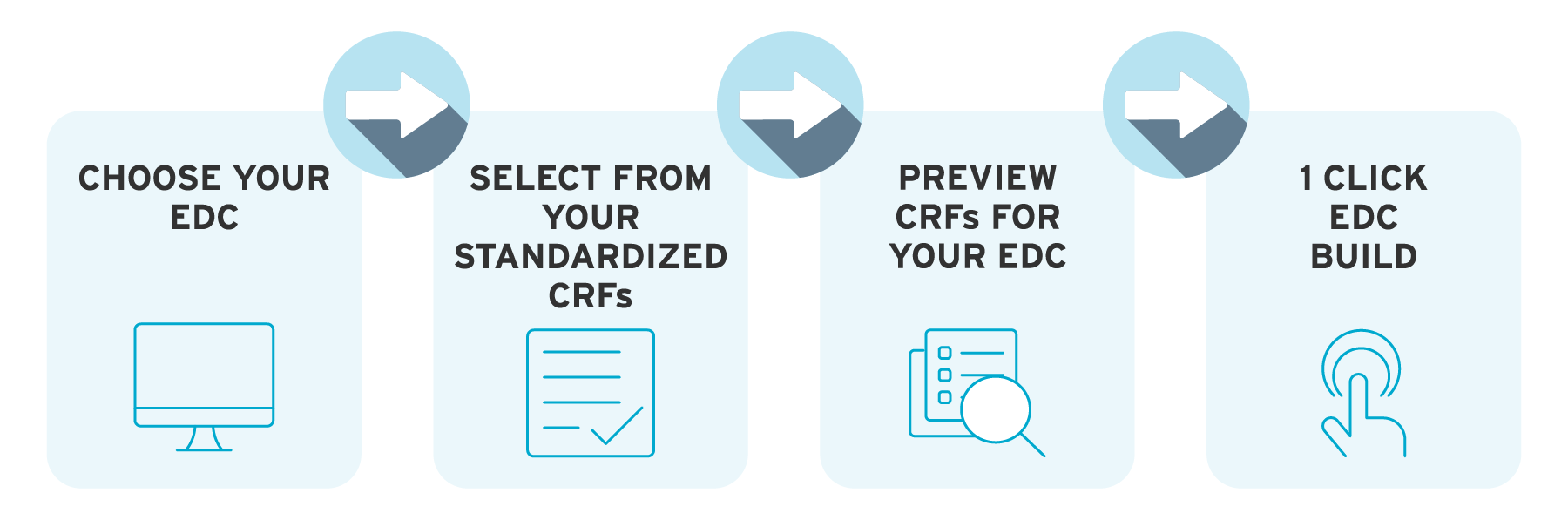
ryze integrates with all the leading EDCs. So you can design, preview, edit, and build your study in ryze. Not only that, ryze validates against the specific rules for your EDC – even if you’re using different systems for study phases.
You can even import study content from your EDC library into ryze. In the case of Rave and Clinical One, our full integration with these systems means the data import and study export back into the EDC can be done in just one click.













Because you can preview and edit CRF designs for your EDC, then instantly see updates in ryze, review cycles can be greatly reduced. One organization we work with has shortened review cycle times from 8 days to just 4.
With ryze EDC integrations, once your study has been approved by stakeholders, it’s just one click to build your study from our eCRF platform.
With a faster, more efficient review-approval process, and automated EDC build, considerable time is saved on study setup. Together with being able to reuse standardized content in ryze, means trials can start far sooner than ever before.
You only need to learn and use ryze to be able to design studies for all the leading EDCs. This particularly saves time and resources for CROs, or for sponsors using different EDCs for study phases.

Organizations can realize huge benefits with ryze visualizations. Our Medidata eCRF tools enable client like argenx to design CRF forms in the platform, and preview how they’ll look and work for their EDC.
Not only does this reduce review-approval times, once forms are approved they can be standardized in ryze and reused across future trials, saving considerable time on study setup.
The reduction in EDC build time is also significant. With ryze, clients like Moderna are able to build studies in half the time – or even less!
Aswell as time efficiencies, organizations can also improve the quality and consistency of studies. Not only can you validate against CDISC standards, ryze also validates against the specific rules for your EDC system.
They increase compliance and increase data quality. There is also better communication. That means less time is needed for data management which results in lower costs.
So that you gather complete and accurate data. They should be well structured, uncluttered, easy to read, and understand. By standardizing study CRFs, your data will be consistent and of a high quality. And you can reuse them. They should address the needs of all key stakeholders such as data entry personnel, the investigator and medical coder.
Yes. Our eCRF tool lets you build eCRFs from scratch, or you can reuse standardized eCRFs. If building from scratch, simply create your form and add sections. Then, add your questions and annotations to it. If you’re reusing a standardized eCRF, you can edit it appropriately. Then in both cases, use our eCRF tool to see what your form will look like in your chosen EDC. Do validation checks and fix any errors. Now, all you need to do is export your eCRF into your EDC.
Yes. You can design your Medidata Rave forms in ryze and visualize how they will look in Medidata Rave. When you are happy with your forms, you can build your Rave EDC at the click of a button.
The ryze metadata repository and clinical trial automation platform will help you design, build, and submit your trials much faster than before.
VAT No. GB 671715037
Company number SC159080