- Book a demo
- Free trial
- Solutions
ryze
ryze FEATURES
Formedix CORE
- Services
- About
- Resources
- Blog
- Contact
- Book a demo
- Free trial

Create ADaM datasets easier and get full traceability of data with ryze
Standardize and reuse your ADaM datasets
ryze clinical trial automation software helps you standardize your ADaM datasets before you’ve collected any patient data. That way, you can check that your datasets will meet regulatory requirements early on. Approved standards can be stored and managed within ryze, so they can be easily found and reused. So, you’ll be able to build studies faster and datasets will be consistent from study to study.
ADaM dataset specifications made easy
With ryze, variables shared between SDTM and ADaM datasets are standardized. This means you can quickly map to your datasets without needing to unpick any data, avoiding delays in the submission process.
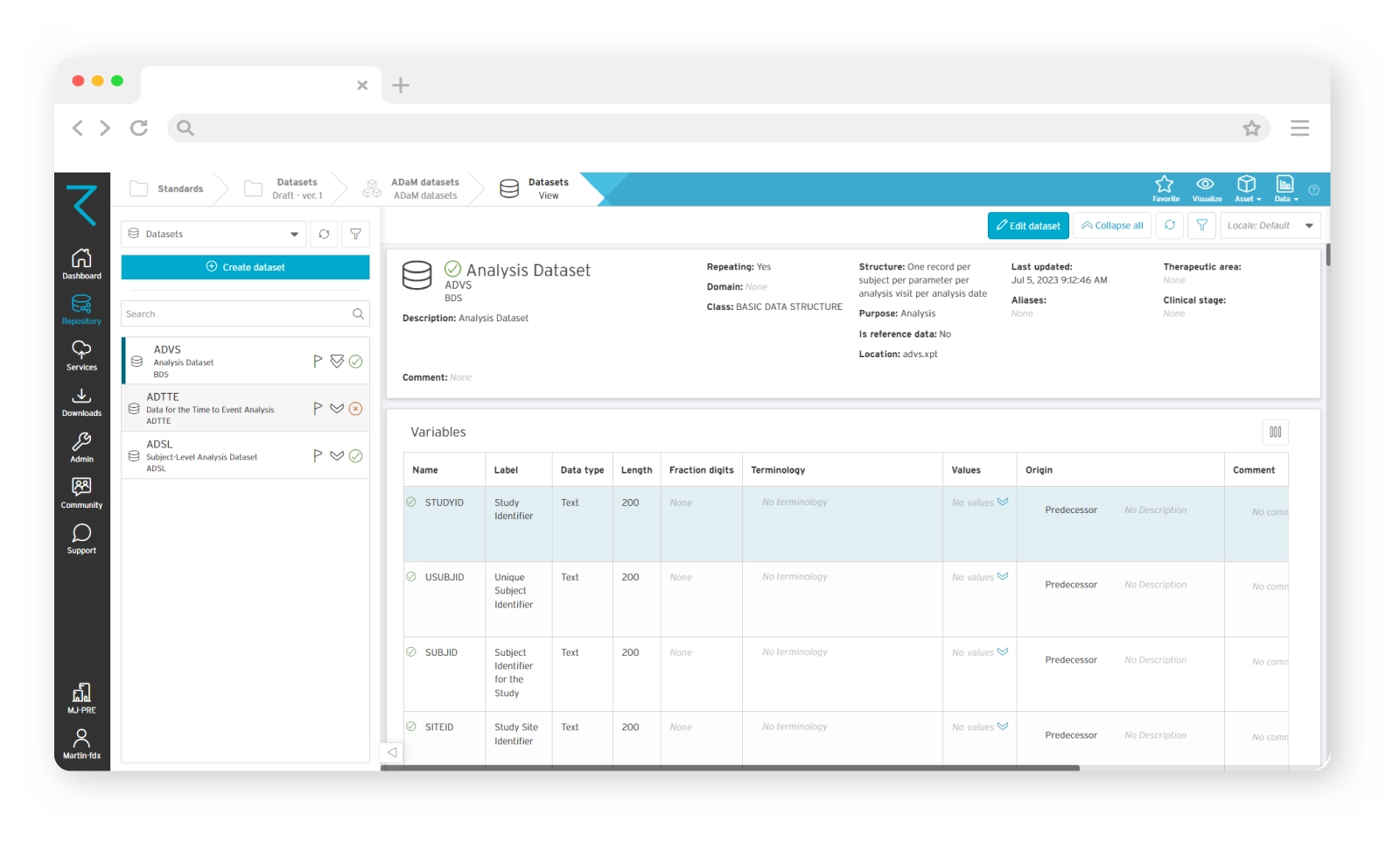
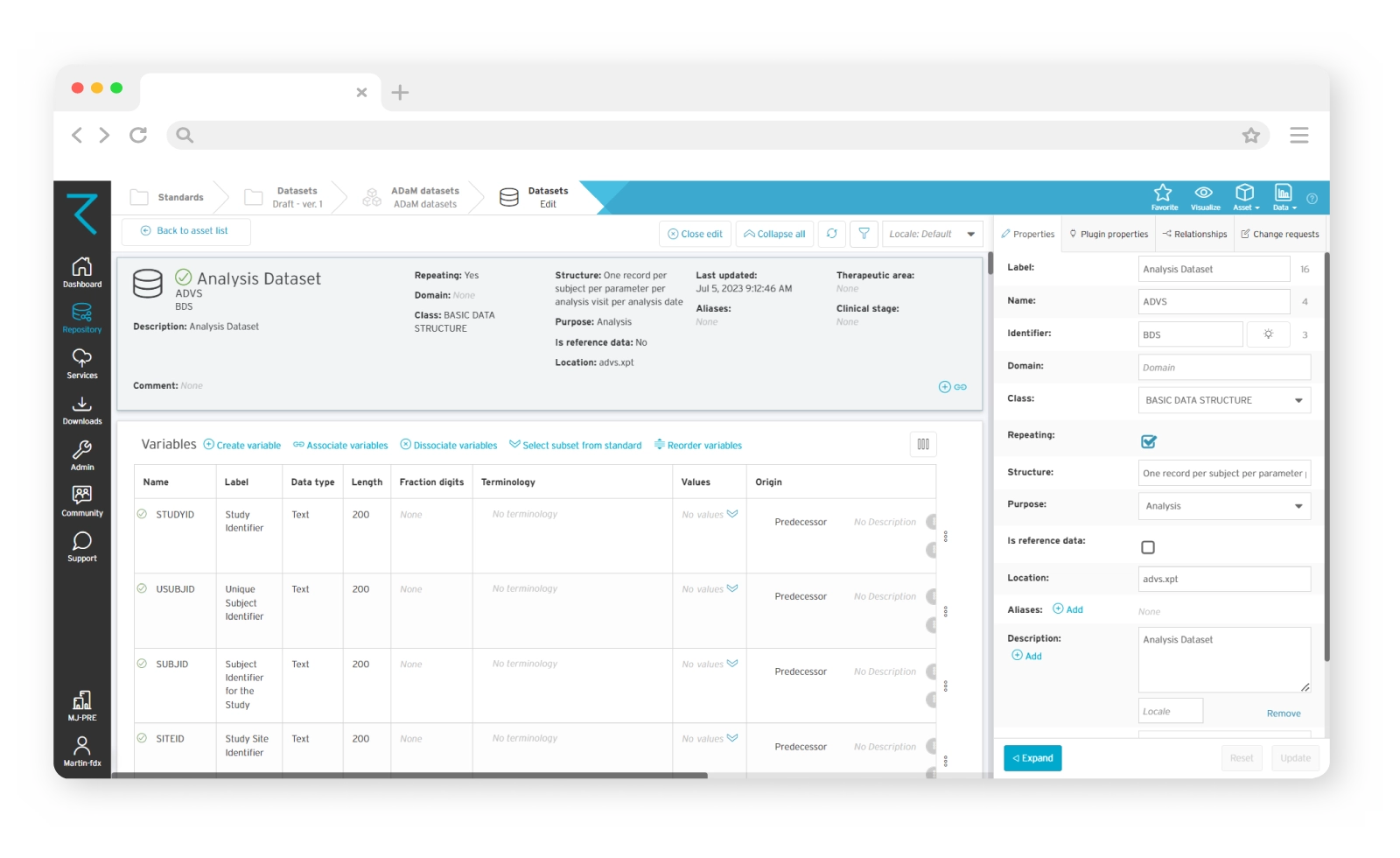
Full traceability of your ADaM data
When it comes to ADaM, traceability is key. It facilitates transparency and builds reviewer confidence in the data itself. Using ryze, you’ll get full traceability of your ADaM datasets, and a clear understanding of the relationship between the analysis results, and the source data. You’ll be able to share standardized variables between datasets, as well as easily see content relationships and the origins of your ADaM data.
Access a built-in standards library
There’s no complex coding in ryze. Instead, you’ll get access to a built-in content library, containing CDISC-compliant published standards. These standards will help you create your ADaM dataset specifications, and give you confidence that they meet regulatory requirements. You can also define your own organization-specific ADaM dataset standards based on these published standards, and build your studies from those.
How you’re better off with ryze
ADaM dataset design
Our clinical metadata management tools help ensure that your datasets comply with CDISC ADaM standards, as well as SDTM, Define-XML, CDASH and SEND. There are several ways to create ADaM dataset metadata in ryze:
SDTM mapping and conversion
ADaM and SDTM go hand-in-hand. Our CDISC SDTM mapping tools let you make and validate simple variable to variable mappings, as well as more complex mappings with many steps.
FAQs
What is CDISC’s Analysis Data Model (ADaM) standard?
ADaM standards outline how to create analysis datasets and associated metadata. This allows a statistical programmer to generate figures, listings, and tables more easily, and ensures traceability. In turn this means that reviewers are able to assess and approve a submission more quickly.
What is the difference between SDTM standards and ADaM standards?
CDISC built SDTM to provide a standard way to organize and format data in order to streamline the processes of collection, management, analysis and reporting of clinical trial data.
While SDTM is used to create and map collected data from raw sources, to ease statistical review, ADaM is all about creating data that’s ready for analysis. ADaM datasets should always be derived from SDTM.














