Complete the form to book your no-obligation demo of ryze clinical MDR and clinical trial automation software… and see how better off you could be with ryze!

Get a free demo of ryze clinical trial software
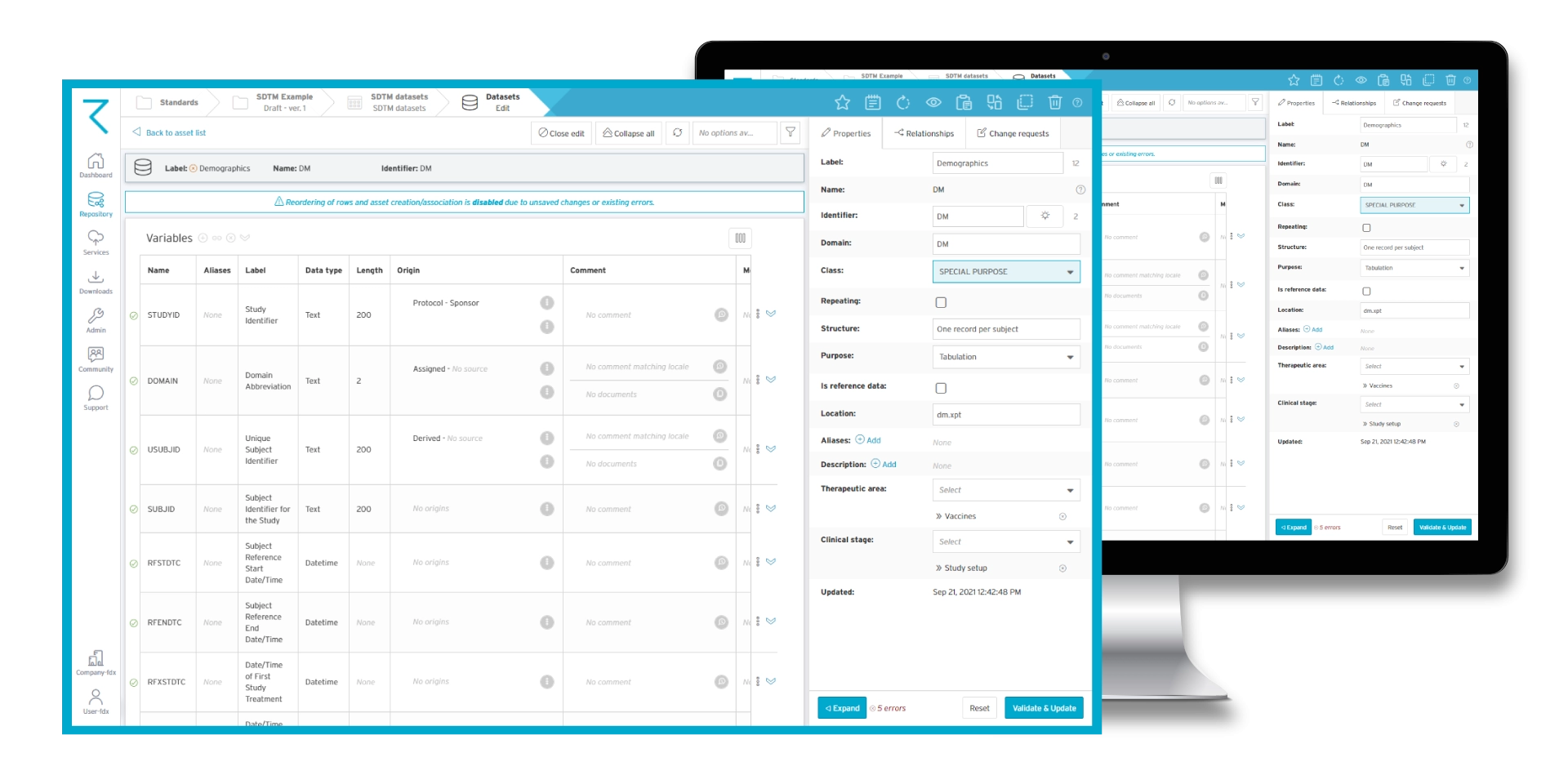
Save time by reusing metadata content in ryze
Design and build CDISC compliant studies in 6 weeks or less
Faster CRF approval cycles
Do SDTM mappings at study design
Get earlier data insights
Organizations using our clinical trial software











What people say about us




VAT No. GB 671715037 | Company number SC159080