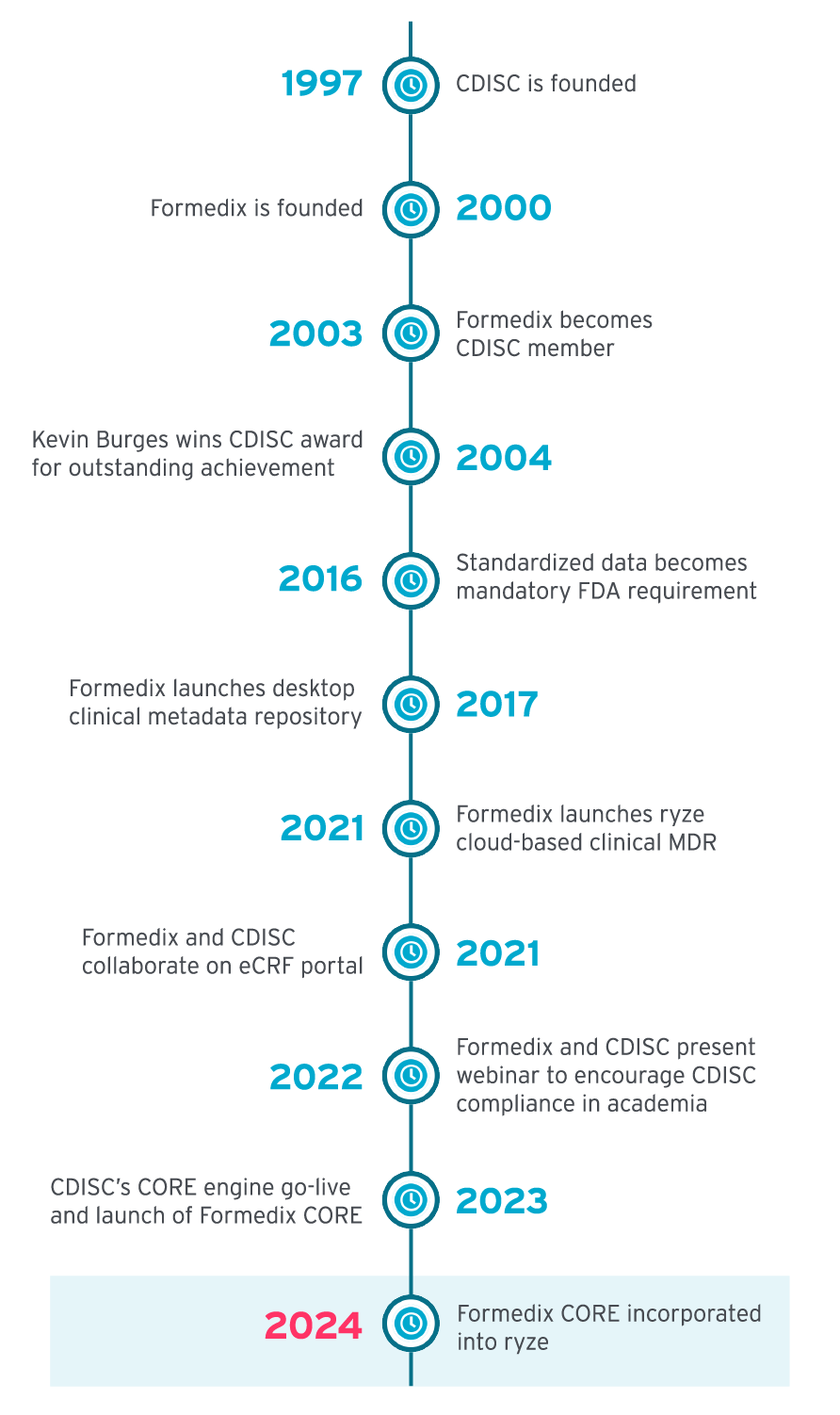
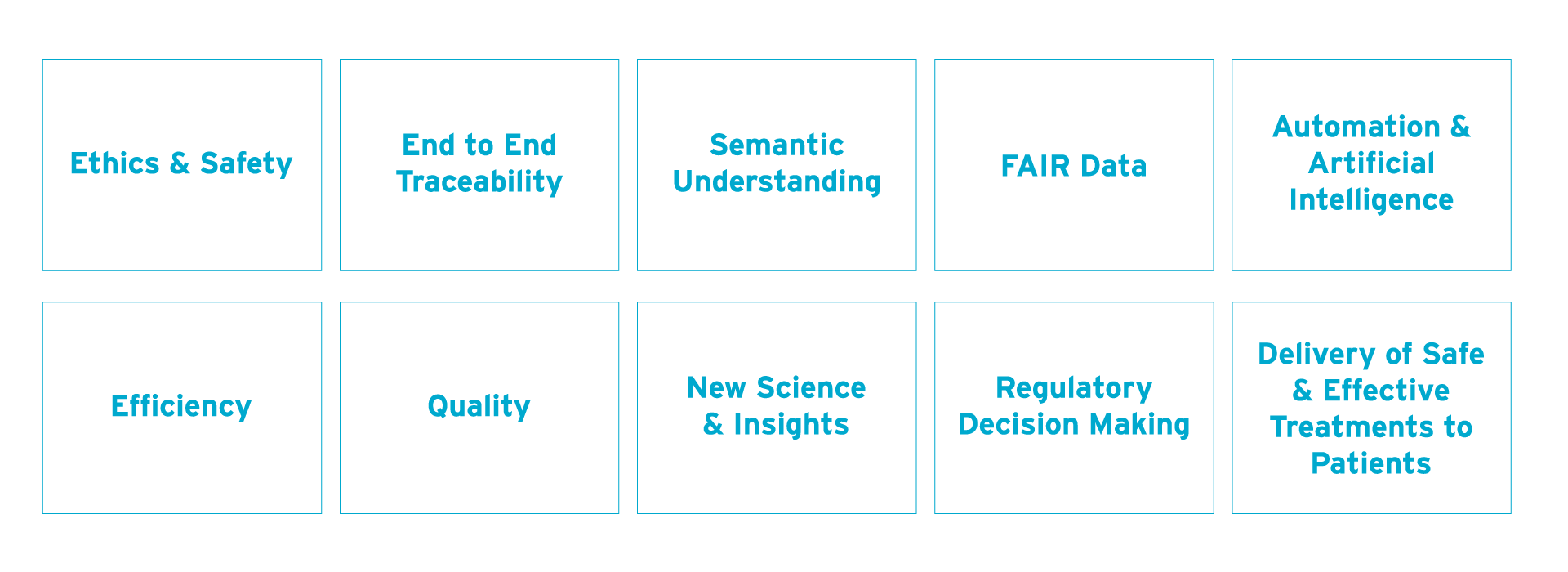
CDISC brought together traditionally siloed functions (i.e., data managers, biostatisticians, clinicians, and terminologists) during the standards development process, which resulted in quality, implementable standards. CDISC encouraged stakeholders to think in advance about the studies they were designing, the outputs, and the best ways to achieve success.
- Book a demo
- Free trial
- Solutions
ryze
ryze FEATURES
Formedix CORE
- Services
- About
- Resources
- Blog
- Contact
- Book a demo
- Free trial