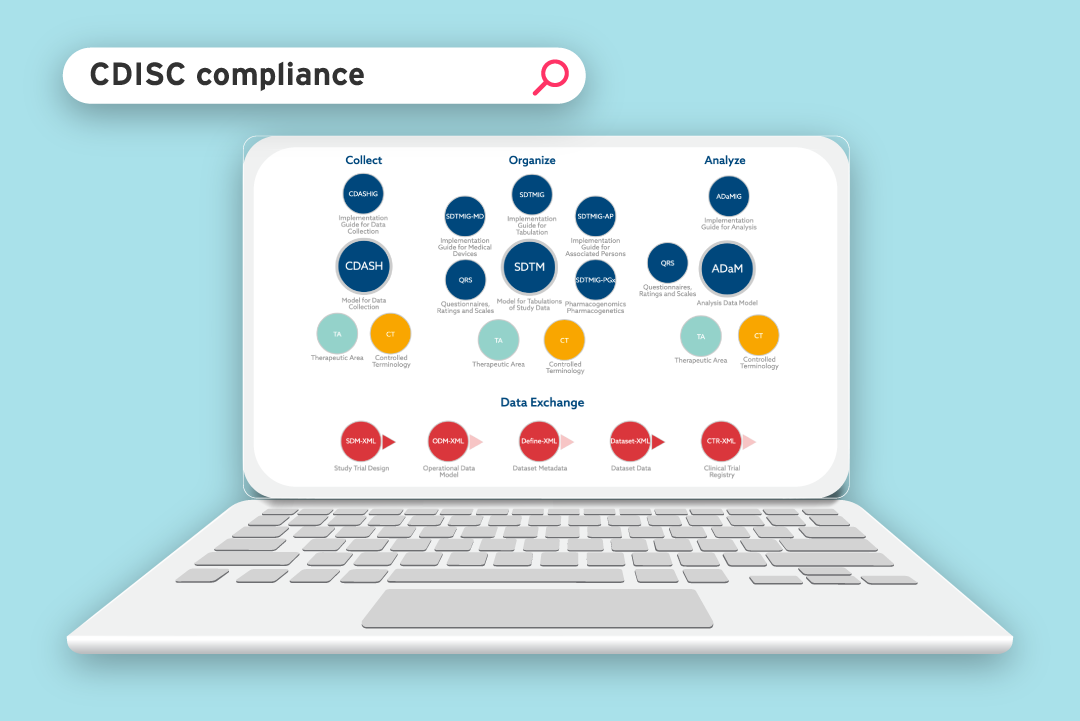
The Clinical Data Interchange Standards Consortium (CDISC) is dedicated to helping improve medical research through data standardization. CDISC has worked closely with the United States Food and Drug Administration (FDA) to introduce data standards, which make it easier for regulatory reviewers to understand and process clinical trial data. Standardized data has been a mandatory FDA requirement for all clinical studies submitted since December 2016.
Now that the pharmaceutical industry is working together using the same standards, clinical trials can be more easily optimized. Data standardization enables the rapid design, build, analysis, and submission of clinical trials. This means new treatments, procedures and tools get to market more quickly, for less cost.
There are many more benefits too:
- Increased efficiency
- Full traceability in the clinical research process from start to end
- Greater innovation
- Better data quality
- Easier sharing of data
- Cost reduction
- Streamlined processes
In this blog, we break down the CDISC standards you need to know about, what they are for, and why they’re important.
Content and Data Exchange standards
CDISC standards cover everything from planning and data collection through to data analysis and reporting.
CDISC standards can be grouped into two different areas:
-
Content standards
-
Data exchange standards
CDISC Content standards
Content standards define what objects are allowed. For example, content standards might define an Adverse Events dataset and the variables it contains.
What content standards should you be aware of?
The content standards used in the clinical research process are:
.png?width=1800&height=400&name=Content-standards-updated%20(1).png)
We discuss each of these standards in more detail below.
The Protocol Representation Model (PRM)
PRM is the very first stage in the end-to-end clinical trial planning process. It’s a conceptual model that’s used to organize the protocol. It identifies items in the protocol and organizes them into a machine-readable structure.
According to CDISC, ’the Protocol Representation Model (PRM) provides a standard for planning and designing a research protocol with focus on study characteristics such as study design, eligibility criteria, and requirements from the ClinicalTrials.gov, World Health Organization (WHO) registries, and EudraCT registries. PRM assists in automating CRF creation and EHR configuration to support clinical research and data sharing.’
PRM is not a deliverable and it’s not as commonly used as other CDISC standards such as SDTM and ADaM.
Clinical Data Acquisition Standards Harmonization (CDASH)
CDASH defines the best way to structure your CRFs to ensure you gather all the relevant data you need for commonly used domains.
CDASH standards help to improve data quality, reduce data queries, and make it easier and more efficient to do the SDTM mappings required for regulatory submission.
 Did you know that CDISC uses the Formedix clinical metadata repository to design and store their eCRF standards?
Did you know that CDISC uses the Formedix clinical metadata repository to design and store their eCRF standards?
Study Data Tabulation Model (CDISC SDTM)
SDTM was developed to organize data collected in human and animal clinical trials. Adhering to SDTM standards helps provide a clear description of the structure, attributes, and content of each dataset, as well as the variables submitted as part of a clinical trial. SDTM metadata is submitted to regulators using the Define-XML data exchange standard.
SDTM was developed so that clinical trial submissions could be standardized and streamlined. Before SDTM, clinical trials differed vastly from each other. For example, there were differences between domain names, variables used, and variable names. This made the review process overly complex and time-consuming. The end result was that it took far longer to get a drug to market.
|
CDISC SDTM consists of 2 parts, the underlying Study Data Tabulation Model and Implementation Guides (SDTM-IGs) that define how the SDTM should be used to represent some common data domains in human clinical trials. |
The core model provides a standardized set of variables, which are grouped into ’classes’. These are refined and built into domains, for example, Vital Signs. The implementation guides serve as a guide for implementing the CDISC SDTM standard.
The latest versions are:
- SDTM v1.7 which supports SDTM-IG v3.3
- SDTMIG-MD (Medical Devices) v1.1
- SDTMIG-PGx (Pharmacogenomics, Pharmacogenetics) v1.0
- SDTMIG-AP (Associated Persons) v1.0
The diagram below shows an example of the different types of CDISC SDTM-related content that contribute to a final SDTM submission.
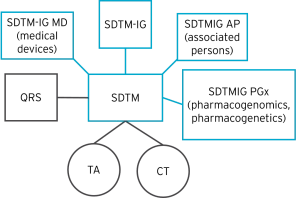
QRS (Questionnaires), TA (Trial Arms), and CT (Controlled Terminology) in the diagram above are covered later in this article.
Wondering about SDTM mapping? Download our free best practice guide to SDTM mapping. Or, if you want to dig a bit deeper into SDTM, check out our blogs on SDTM supplemental qualifiers and LOINC codes for SDTM.
Standard for Exchange of Non-Clinical Data (CDISC SEND)
SEND standardizes the exchange of non-clinical data between systems in a consistent format. SEND metadata is submitted to regulators using the Define-XML data exchange standard. It’s required by the FDA for submissions. It’s an implementation of the SDTM standard that’s used for animal studies. In other words, data that is collected for animal studies can differ from data that is collected for humans, and the SEND standard attempts to fill this gap.
The Analysis Dataset Model (CDISC ADaM)
The CDISC ADaM standard was developed to standardize analysis data. It ties in very closely with SDTM. While SDTM is for collected data, ADaM is for presenting analysis data. Adam datasets must always be derived from SDTM datasets. ADaM metadata is submitted to regulators using the Define-XML data exchange standard, and also the related Analysis Results Metadata standard.
The characteristics of the CDISC ADaM standard are as follows:
- It’s submitted using Define-XML
- It’s more flexible than SDTM
- Traceability is built-in among analysis results, analysis data, and data represented in the SDTM
- It fulfils most data analysis requirements
Like SDTM, the ADaM standard also has an implementation guide.
The latest versions are:
- ADaM v3.1
- ADaM-IG v1.3
Read our blog on 3 things you should know about ADaM standards.
The diagram below shows an example of the different types of CDISC ADaM-related content that contribute to a final ADaM submission.
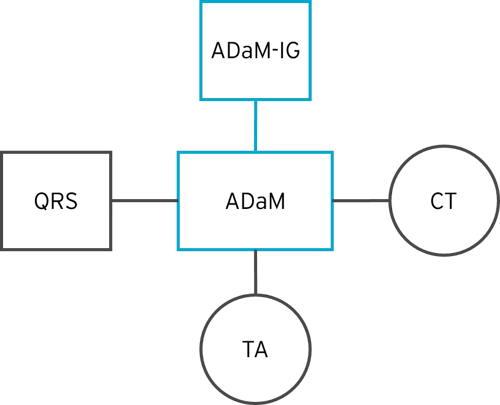
Questions, Ratings, and Scales (QRS)
QRS instruments are questions, tasks, or assessments for qualitative and quantitative assessment for clinical trials. A questionnaire is a series of related questions that produce one or more scores. Ratings are a ranking of quality, standard, or performance. Scales are defined based on criteria that result in a single measurement.
The QRS team develops Controlled Terminology and SDTM supplements, while the ADQRS team develops ADaM supplements. CDISC creates supplements for questionnaires, functional tests, and clinical classifications. These supplements provide standards for collecting and storing responses from QRS.
Some examples of published supplements include the 6-minute walking scale, Hamilton anxiety rating scale, and the Neuropathic pain scale.
Controlled Terminology (CT)
CDISC partnered up with the National Cancer Institute (NCI) to publish an evolving set of terminology standards. These are used along with content standards like SDTM to help ensure that the content of the data is easy to understand and is consistent.
Controlled terminology is a set of code lists and valid values that are references by questions in forms and variables in datasets. In a nutshell, it tells you how you should submit collected data for a data item in a dataset. For example, if a question on the CRF is to record sex, the allowed values are F (female), M (male), U (unknown), or UNDIFFERENTIATED.
Controlled terminology is used for PRM, CDASH, SDTM, SEND, and ADaM standards.
You can read more on NCI CT in our blog about using NCI controlled terminology for standardizing data.
Therapeutic Area Standards (TA)
Therapeutic area standards are an extension of the foundational standards. They serve to represent data for specific therapeutic areas such as asthma, diabetes, multiple sclerosis, and many more.
Therapeutic Area User Guides (TAUGs) are implementation guides for each specific area. The CDISC foundational standards define in general how each different type of data should be submitted, and the TAUGs provide more specific information about how to interpret the foundational standards within a particular therapeutic area. For example, they might define how a variable should be interpreted in the context of a specific TA.
CDISC Data exchange standards
Data exchange standards are ways of representing metadata and data in a standardized way in order to make it easier to exchange data between different parties. They define how you describe an object.
Data exchange standards described in this article include:
.png?width=1800&height=360&name=Data-exchange-standards-updated%20(1).png)
We discuss each of these standards in more detail below.
ODM-XML
The CDISC Operational Data Model (ODM) is an XML-based model for standardizing the transfer of metadata for clinical trials and the associated data. It can be used for defining the data collected in a trial, such as CRFs and patient diaries, to provide an upfront specification for the trial. This can then be used to help automate the build of the data collection systems. It can also be used for transferring the data itself once collected.
The latest version is ODM-XML v1.3.2.
Some other models such as Define-XML, Dataset-XML, and Analysis Results Metadata are implemented as extensions to ODM-XML.
By using ODM and CDASH together, you can rapidly define the data you need to collect in your clinical trials. This ultimately helps to reduce the time it takes to get a drug to the market.
Dataset-XML
Dataset-XML supports the exchange of tabular data using ODM based XML technologies. It allows communication of study datasets for regulatory submissions.
Define-XML
Define-XML is a data model that allows a standardized description of tabular dataset structures, helping to drive process efficiencies throughout the clinical lifecycle. Simply put, it describes the structure and content of data collected or submitted during the clinical trial process. This includes system-specific dataset structures that are exported from an EDC system and standardized CDISC domains such as SDTM.
It’s an extension of the CDISC ODM standard and is a key requirement for describing datasets that are to be electronically submitted to the FDA and PDMA.
Define-XML 2.1 is the current version of the standard.
Read more about using Define-XML for dataset design and how to describe multiple origins for a value in Define 2.0.
Analysis Results Metadata (ARM)
CDISC standardized the description of ARM for describing Tables, Listings, and Figures (TLFs). This references the data in standardized ADaM datasets, making it easier to re-use analysis results metadata across different studies.
Laboratory Data Model (LAB)
Lab was established to standardize the transfer of data between clinical laboratories and sponsor companies. The CDISC LAB model is widely used in pharmaceutical and biotechnology companies today.
It was developed because of the many variations between data acquired in laboratories, such as lab test names and units. So, by using the LAB model, laboratory data is standardized allowing seamless transfer between laboratories and CROs, which saves time and costs.
What CDISC standards are required for submission?
The FDA and the Japanese Pharmaceuticals and Medical Devices Agency (PMDA) require the following standards:
- SEND (Standard for the Exchange of Nonclinical Data)
- SDTM (Study Data Tabulation Model)
- ADaM (Analysis Data Model)
- Define-XML
- Controlled Terminology
Additionally, CDISC standards are the preferred standards for electronic submissions to the Chinese National Medical Products Administration (NMPA).
CDISC Library
The CDISC library is a central repository for developing, integrating, and accessing CDISC metadata standards. In other words, it’s an online electronic source for the CDISC content standards, allowing them to be viewed in a machine-readable way. It makes it easier for users to implement CDISC standards via clinical trial software such as a CTMS (clinical trial management system).
The library provides an API that helps to automate the implementation of CDISC standards. Users can access standards in real-time in a number of different formats. For example, RDF, XML, JSON, and CSV.

Need help with CDISC standards?
Formedix have been strong advocates for the use of CDISC data standards in clinical and non-clinical research and are industry leaders in CDISC-compliant software. In fact, we were one of the first ever CDISC members over 18 years ago. Today, we’re active members of the CDISC XML Technology team, and our friendly experts regularly speak at industry events.
And because ryze, our clinical MDR and clinical trial automation suite, is built on the latest CDISC standards, it’s safe to say we know our stuff!
To learn more about the CDISC standards required for regulatory submission, download our free guide by clicking the button below.
Author's note: this blog post was originally published in January 2021 and has been updated for accuracy and comprehensiveness.
![]()
About the author

Gilbert Hunter
Customer Success Manager | Formedix
Gilbert joined Formedix nearly ten years ago as a technical writer. The system knowledge he gained from content development, together with his existing customer service skills, marked him out for transition to the Professional Services (PS) team.
Gilbert has worked with the PS team for over four years, providing both CDISC-based training and software training, as well as support and consultancy services to Pharmaceutical, Biotechnology and Clinical Research Organizations. He helps organizations build studies faster and to a higher quality by making their clinical trial design and regulatory submissions far more efficient.
Today, as Customer Success Manager, Gilbert’s focus is to ensure customers maximize the benefits they can achieve from ryze by overcoming their challenges and achieving their goals.