Standardize and reuse approved metadata content in ryze Clinical Metadata Repository (MDR)
- Book a demo
- Free trial
- Solutions
ryze
ryze FEATURES
Formedix CORE
- Services
- About
- Resources
- Blog
- Contact
- Book a demo
- Free trial
Standardize and reuse approved metadata content in ryze Clinical Metadata Repository (MDR)
ryze provides a centralized clinical metadata repository to store, share, manage and reuse standards and studies throughout a trial’s lifecycle. Not only that, you can transfer metadata between studies, and drive automation downstream by integrating with EDC and other clinical systems. As a result, we’ve seen organizations reduce study setup time from 15 days to just 2, and review cycles shortened from 8 days to 4.
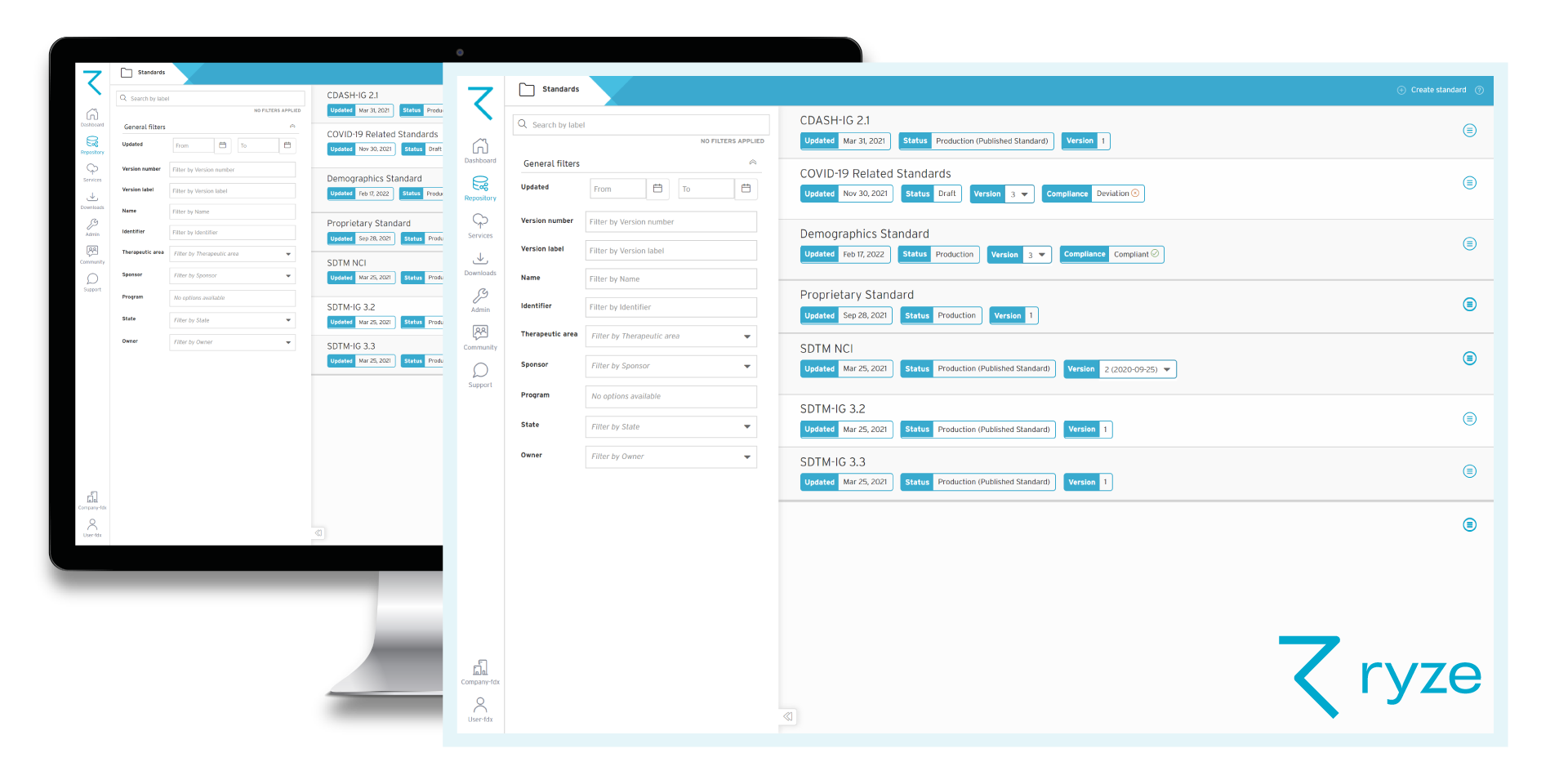
Having standardized content in a clinical metadata repository enhances data quality and consistency, since you’re reusing pre-approved, accurate, validated content across future studies and standards.
With clinical metadata being managed in one central place, teams can easily find, share and collaborate on content – knowing that they’re using the correct, most up-to-date version of a standard for example.
Standardized metadata in your MDR already aligns with CDISC and meets regulatory requirements – it’s previously been validated and approved! And when you create new content, you’ll design with CDISC compliant templates, and be prompted to fix any compliance issues as you go.
You’ll have full visibility of all changes made to studies, standards, and other metadata over the lifecycle of the trial. Plus, you can track how data flows from the CRF right through to raw datasets, SDTM and ADaM.
You’ll see the impact of making proposed changes to standards in your MDR before you make them. That way you can clearly see the implications and make informed decisions about whether to proceed.
Easily control and manage metadata end-to-end with an integrated governance lifecycle in your MDR. Use built in approval processes for creating standardized content, and define relevant lifecycles for standards and studies to control how metadata can be used.
Building studies is far quicker with standardized, pre-approved, validated content in your clinical metadata repository. Plus, you can instantly build a full study for your EDC from standards in ryze! So you could have your study built and ready to start trials far earlier – we’ve seen organizations reduce study build from 15 days to just 2 after standardizing metadata in ryze MDR.
Building studies from standards means less time and resources are needed for study setup. This equates to significant savings in study build costs – especially over multiple trials. Coupled with that, faster study launch accelerates the process to submission. And the sooner you gain FDA approval, the sooner you can bring profitable products to market.
Put yourself in the best possible position for getting submissions approved. The improved quality you’ll gain through having standardized content that complies with CDISC and FDA regulations, will boost your chances of a successful submission.

Watch the webinar to hear how global biotech, argenx, introduced standardization, and how this impacted trial setup.
A huge benefit of standardizing clinical metadata in your MDR is that you can instantly build studies for your EDC from a set of standards in ryze.
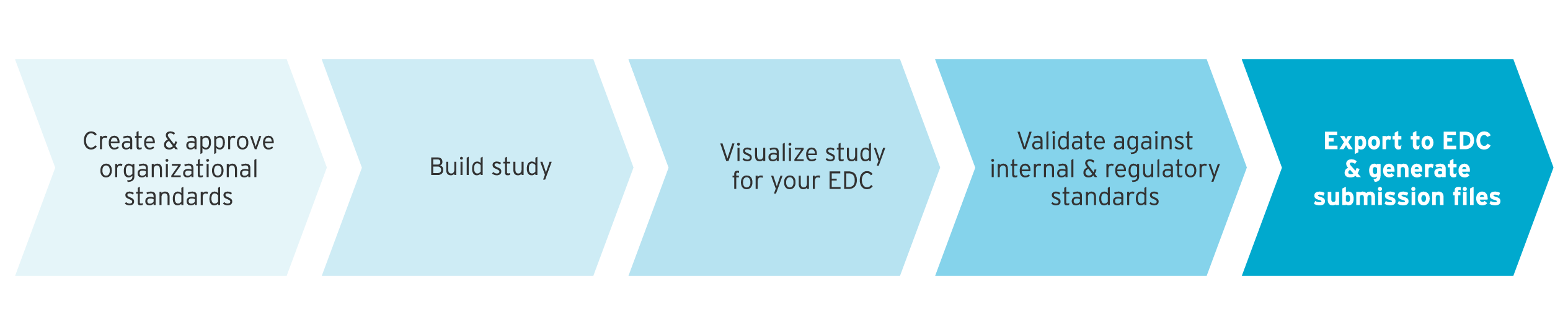
First, create and approve organizational standards using a company defined governance workflow in your MDR. This ensures that a consistent protocol is followed, and provides complete control across the study design process.
Choose the required forms and datasets from standards to build your study. All related metadata, such as edit checks and mappings, are automatically included with your standards.
Add any relevant protocol information, such as visit schedules, custom edit checks, or custom mappings. Then use ryze visualization tools to see exactly how CRFs look and work for your EDC.
Validate your study to ensure there are no compliance errors or missing metadata.
Finally, export your study to the relevant EDC system. Now you can generate submission deliverables, including Define.xml, as well as downstream datasets from mappings in your study design.
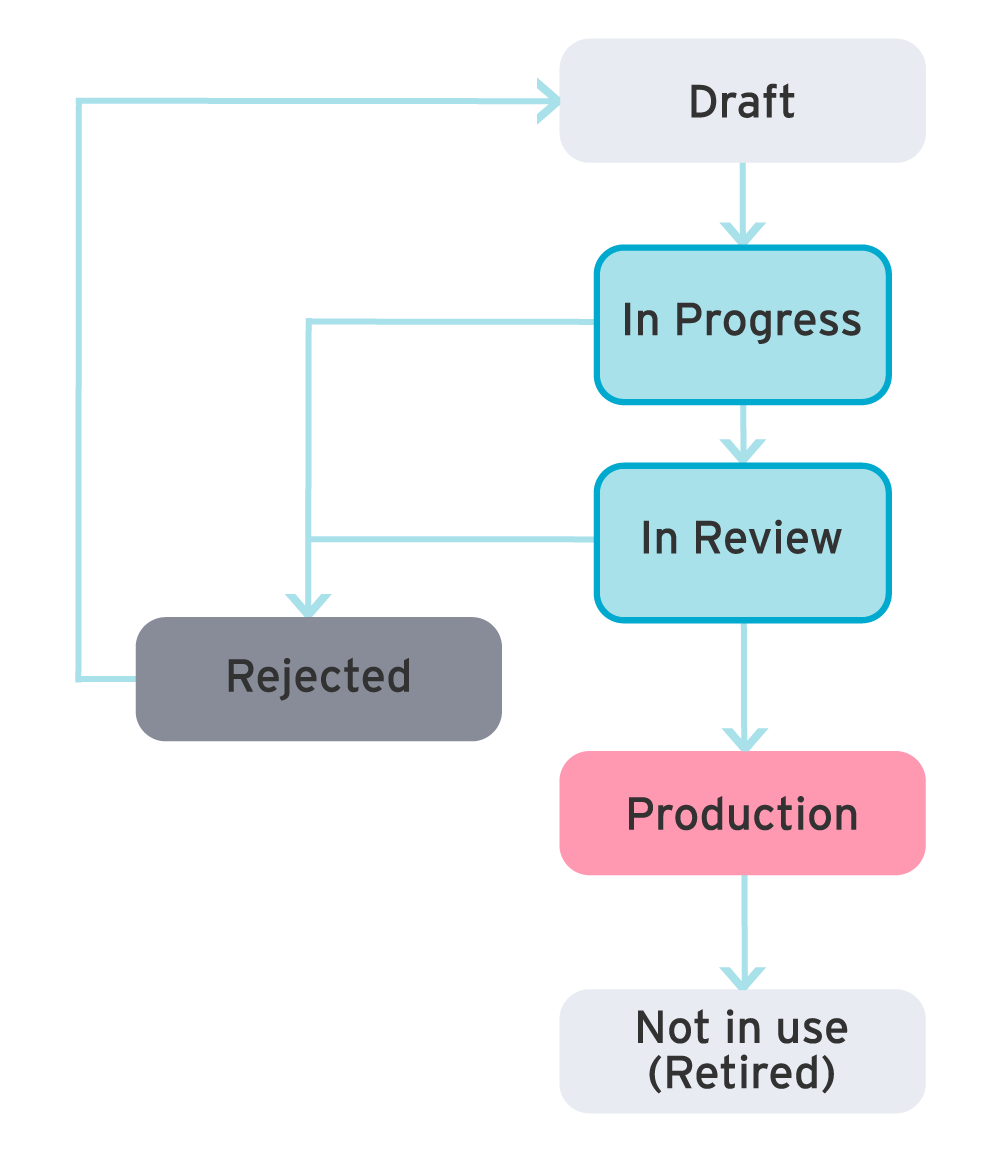
No problem! In ryze, metadata lifecycles can be customized according to an organization’s governance process. For example, you may need separate lifecycles for studies and standards. New lifecycles may be added, cloned or removed. And approval states can be added, edited or removed.
Governance and change management is key to the evolution of your organizational standards. With ryze, governance is built into the platform from launch, in order to facilitate more robust, consistent and higher quality standards.
To allow you to create and manage change requests, measure the impact of changes and have governance built in. They should also allow collaboration among teams and be able to integrate with other systems.
To be CDISC complaint, stay up to date with new standards and support older versions of standards. They should also allow reuse, have versioning, built in traceability and allow you to find metadata quickly and easily.
Yes. Using the ryze clinical metadata repository means you have standardized content ready to use again and again across your studies and standards. It’s quick and easy to make updates. Once your happy, it’s a click of a button to create your clinical study build.
The ryze metadata repository and clinical trial automation platform will help you design, build, and submit your trials much faster than before.
VAT No. GB 671715037
Company number SC159080