The hard work’s already done. Now you’re just 1 click away from define.xml
- Book a demo
- Free trial
- Solutions
ryze
ryze FEATURES
Formedix CORE
- Services
- About
- Resources
- Blog
- Contact
- Book a demo
- Free trial
The hard work’s already done. Now you’re just 1 click away from define.xml
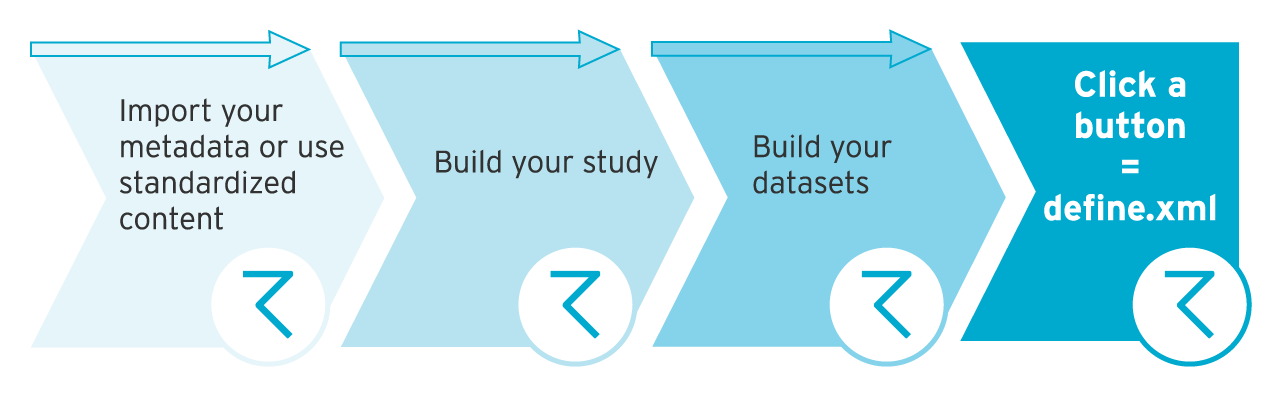
Once you’ve finished your study, it’s all about FDA submission. And that means having your study metadata in STDM define.xml format. Unless you’ve got the right programming skills, this can be a really daunting and time consuming process.
The quick way? Create the relevant define files with ryze visual define xml editor. Once your metadata is in our library, you’ve built your study and defined your datasets, you can create define.xml file in 1 click. No need to understand code or XML. And if you’ve got a spreadsheet, just import it into ryze to convert it. You can even convert a SAS XPT file or old legacy datasets. See how our visual define XML editor gives you faster define.
Want to create define.pdf file for your FDA submission? Just click a different button. Not only do you save time at the end of your study, you have the chance to get your study submitted much sooner too.
Create SDTM define.xml files at the click of a button in ryze. Our platform has a visual define XML automation tool built in so there’s no manual work involved. And you don’t need to be a SAS expert. Instantly convert Excel or SAS XPT files to define.xml, define.pdf, or define.html with our visual XML editor.
Because you create define files much quicker, you can be ready to submit your study to the FDA much sooner.
Because we’ve built the Define-XML standard into ryze, you automatically comply with the rules. You’re using a platform that’s built on the latest FDA and NCI standards. Plus, you’re covered for older standards – we still support those too.
Our validation tools help you check your datasets, and spot any deviations from Define-XML standards. So when you’re ready to create your define file for submission, you can be confident that it complies.
Instantly make SDTM define.xml and ADaM-based define.xml from your ryze datasets.
The Define XML standard is built into our platform. That means you’ll automatically comply when designing your metadata. So less effort is needed in the future to create define compliant metadata – compliance is built into your dataset design by default.
SDTM define.xml is a document that describes the structure and content of data collected during a clinical trial and is required for a regulatory submission. The SDTM Define standard supports the submission of clinical trial data in CDISC SDTM for regulatory submissions.
The FDA requires all submissions to use Define XML to describe datasets. In ryze, you can quickly define your SDTM, SEND and ADaM datasets upfront using the visual define.xml editor. And you’ll be confident knowing your submission will be compliant.
Yes, our visual define.XML editor lets you do this. And it’s easy to understand the content of datasets and use them appropriately.
They are used to specify when a specific Value definition applies.
By using “where clauses”. You need to create 2 values with the same name – however they must have different OIDs. Find out more about Describing Multiple Origins for a Value in Define-XML 2.
The ryze metadata repository and clinical trial automation platform will help you design, build, and submit your trials much faster than before.
VAT No. GB 671715037
Company number SC159080