But it doesn’t have to be this difficult… with the right technology you can be a clinical study pro and master clinical trial setup!
So what do the best clinical study pros do?
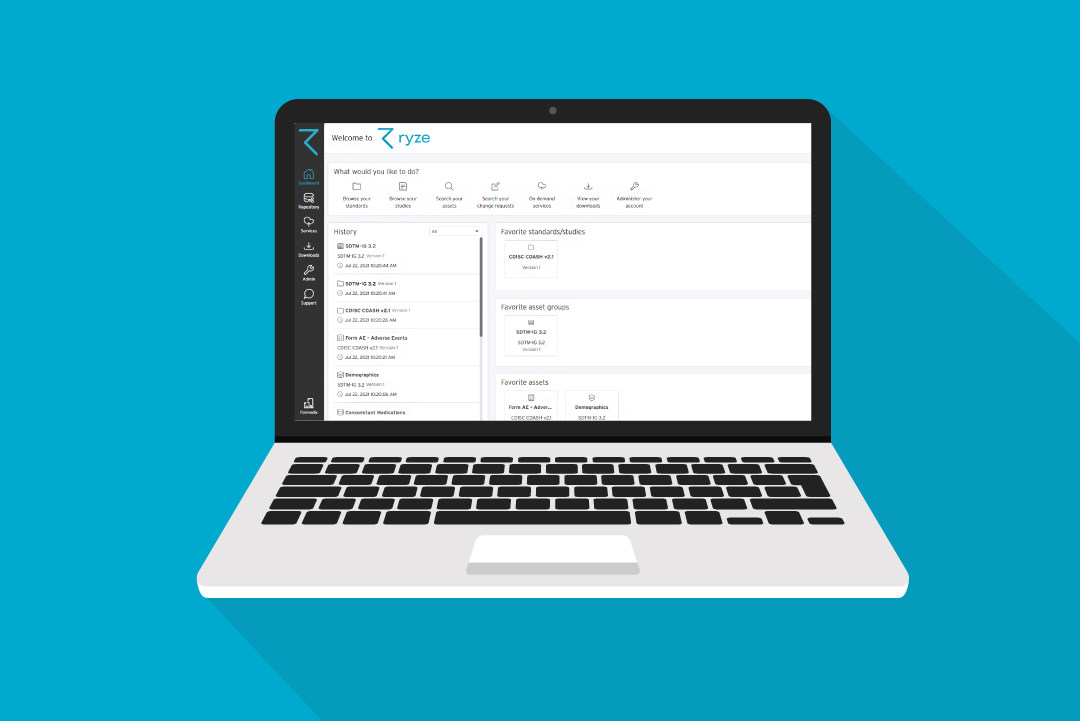
They use Formedix ryze!
While we can’t help with changes to your protocol, our cloud-based platform ryze, can help with pretty much everything else.
You can build content from scratch quickly in Formedix ryze. Or you can import from standard libraries, or directly from EDCs like Rave and InForm.
It’s one click to preview your annotated forms for the EDC of your choice. Plus you can validate your studies as you go. And once you’ve done all that, it’s only one click to build your study in ryze. It’s all validated and ready to export to your chosen EDC!
1 click clinical study build. Really?
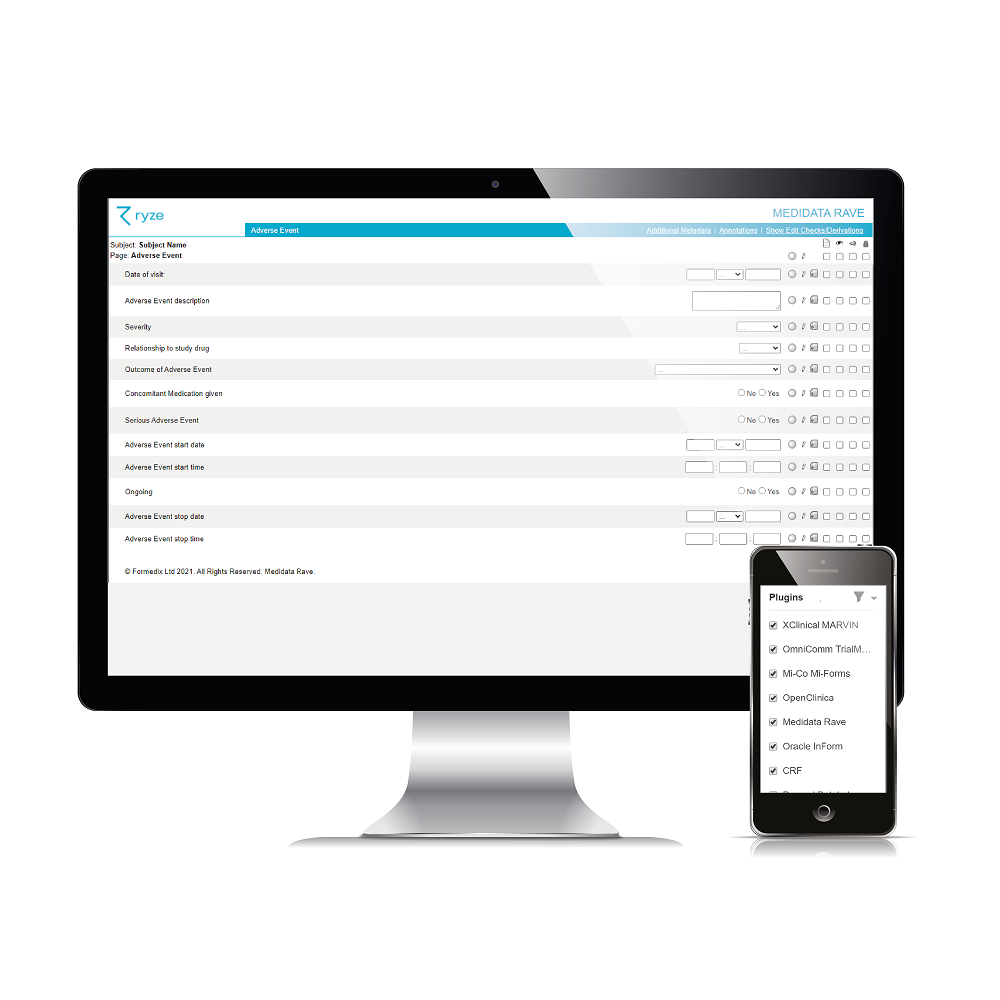
It’s easy to see what CRF designs and annotations will look like in your chosen EDC system, before you build your study, so you can get them look perfect before you even start your study! Literally one click to preview is all it takes.
Note:
When everything’s all set up, one click and hey presto! Your study is built and ready for export!
Are you starting to see how much time you could save? Find out more about building and previewing your EDC study in ryze.
What about integration with EDCs?
Formedix integrates with 7 leading EDC systems. Simply choose the one (or more than one!) that you want to build a study for. And you can even import studies from Rave and InForm into Formedix.
And validation?
Validation couldn’t be easier. By default ryze automatically validates your study against CDISC ODM. After you’ve chosen your EDC, run the validation tool, and fix any errors. It’s quick and easy as you know exactly where to find them in your study.
Can study content be reused?
You can reuse your standardized study content. Just choose what you want to import into the ryze clinical metadata repository platform. It could be the whole study, organizational standards that you want to manage. It could be forms, spreadsheets (including content), or even just some terminologies. It’s up to you! That way you have consistent, validated content. And you can reuse it as often as you like.
You can also reuse your edit checks. They can be a real pain and take quite a bit of time to set up. Because edit checks can be similar across studies, it makes sense to reuse them. Why make more work for yourself? All you have to do is import them, edit them to suit, and that’s you done!
How quick is it to get up and running?
Well, this is going to vary from organization to organization. But, we estimate that if it usually takes you 20 weeks to make a clinical study build, you can do it in as little as 4 weeks with ryze. That’s what makes ryze users clinical study pros!
Get your source define-XML
After setting up your clinical study, another benefit is that you can create your source define-XML. There’s no worries with it meeting regulatory requirements when you submit your study. It’s hassle-free! Mappings are part of the study design so you can also create downstream datasets. And you don’t have to program the data conversion for your study. That’s more time saved!
Check out the main benefits of ryze in the infographic below!
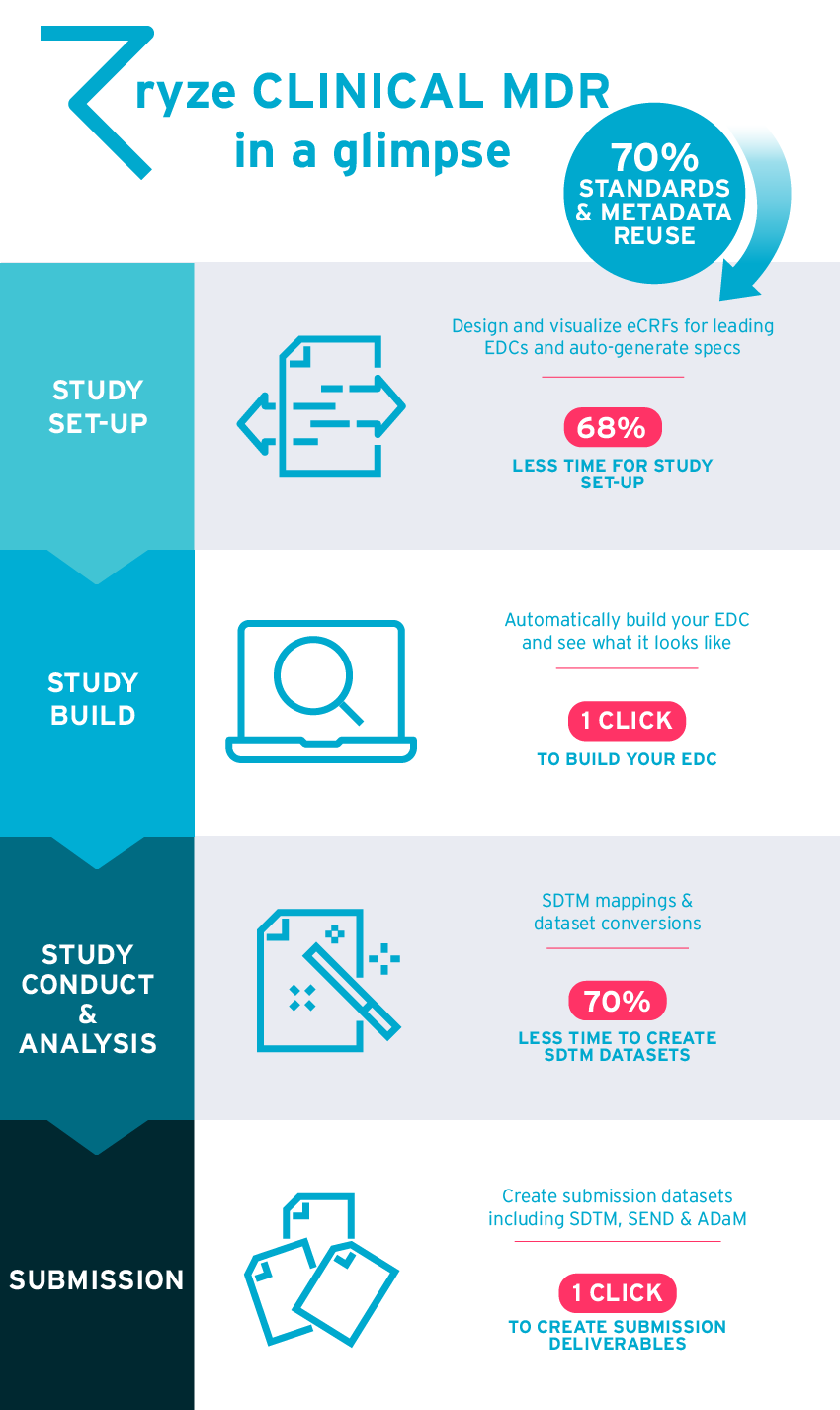
Become a clinical study build pro
We’re constantly expanding and enhancing our platform. So, you’ll always be on the right side of CDISC compliance. Plus, you’ll have a regular flow of new features that help you do even more things, quicker and easier. The bulk of our development is based on customer feedback. Just pass on your wish list and we’ll see what we can do!
Are you seeing how easy it is to become a clinical study build pro with ryze? Get in touch and we can arrange a free no-obligation demo. Or if we’ve whet your appetite and you’re keen to see it for yourself, we offer a free 30-day trial of ryze with 6 hours free training!