- Book a demo
- Free trial
- Solutions
ryze
ryze FEATURES
Formedix CORE
- Services
- About
- Resources
- Blog
- Contact
- Book a demo
- Free trial
- Book a demo
- Free trial
- Solutions
Formedix CORE
ryze
ryze FEATURES
- Services
- About
- Resources
- Blog
- Contact
- Book a demo
- Free trial
-
![]()
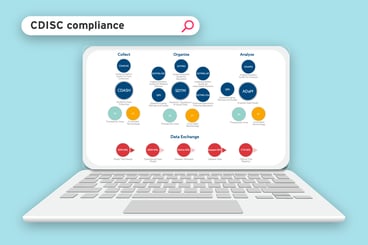
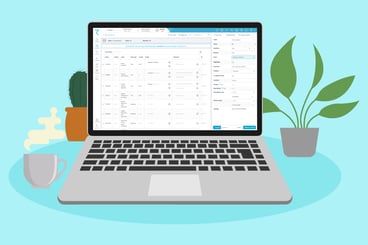
The ryze metadata repository and clinical trial automation platform will help you design, build, and submit your trials much faster than before.
VAT No. GB 671715037
Company number SC159080