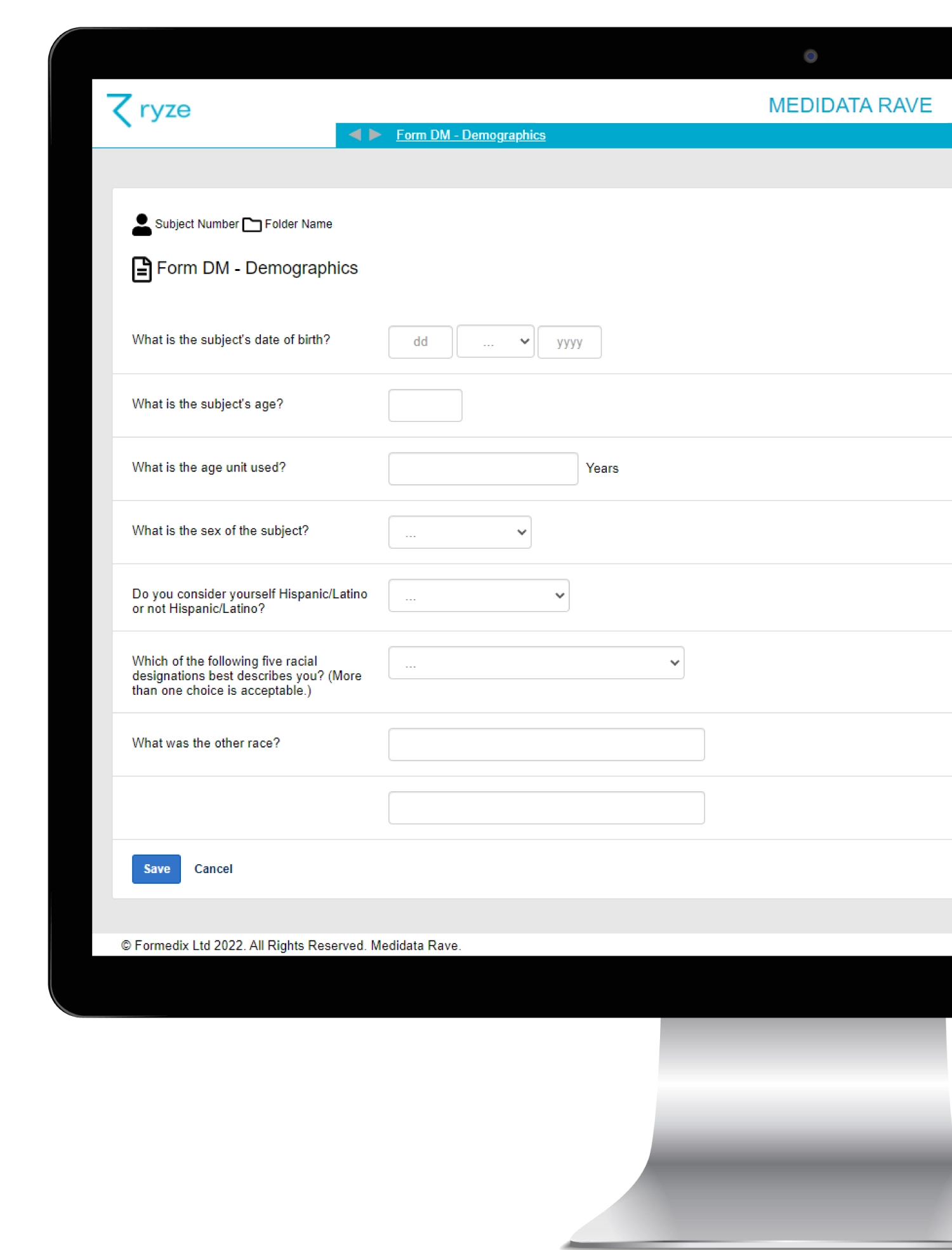
Whether you’re using multiple EDC systems or just one, ryze clinical metadata repository can help you design and visualize how your EDC will look – before it’s built! You can also store standardized study content in ryze, and easily build your EDC from your approved standards.
In this step-by-step guide, we’ll show you how to easily design your EDC in ryze, and start building faster, more efficient clinical studies.
Download the guide now!